China: researchers uncover exceptional barocaloric effect enabled by dissolution of NH₄SCN
A team of researchers led by Prof. LI Bing from the Institute of Metal Research of the Chinese Academy of Sciences have introduced barocaloric effect (BCE) at dissolution, a new refrigeration concept which offers larger cooling capacity than existing barocaloric systems and efficient heat transfer comparable to that of conventional vapour compression systems.
According to the UNEP’s Global Cooling Watch 2025, cooling demand could more than triple by 2050, which would almost double emissions over 2022 levels [1]. Low carbon alternatives to conventional vapour compression refrigeration are therefore urgently needed.
Solid-state refrigeration systems offer the potential for low-noise, compact, and environmentally friendly alternatives to traditional systems.
What is Barocaloric Cooling?
When a barocaloric material is exposed to an increase in hydrostatic pressure, it generates heat that can be absorbed by the heat transfer fluid. Then, upon decompression, the barocaloric material cools down and absorbs the heat from the heat transfer fluid [2].
In 2016, colossal barocaloric effects were discovered near room temperature in organic materials made from cheap, abundant elements [3]. For the first time, these materials matched the performance of hydrofluorocarbons and hydrocarbons used in today’s heat pumps, refrigerators, and air conditioners. Developments in barocaloric-based refrigeration and heat pump technologies could therefore contribute to achieving near-zero emissions from refrigeration [3].
Innovative Extreme Barocaloric Effect
A team of researchers led by Prof. LI Bing from the Institute of Metal Research of the Chinese Academy of Sciences have introduced a new refrigeration concept: barocaloric effect (BCE) at dissolution, which uses pressure-tuned dissolution heat.
The effect was demonstrated using ammonium thiocyanate (NH₄SCN) dissolved in water. Inexpensive and readily available, NH₄SCN was the first reported system to exhibit a colossal barocaloric effect. In their study published in Nature, researchers discovered an exceptionally strong thermal response arising from its pressure-tuned dissolution. In other words, the dissolution releases heat, and applying pressure causes the salt to precipitate, enabling a reversible cooling cycle (see figure 1). Moreover, the aqueous solution simultaneously serves as a direct heat-transfer fluid. This dual functionality closely resembles that of conventional vapour refrigerants and allows efficient heat transfer.
Experimental results showed a temperature drop of 26.8 K in the solution at room temperature under 600 MPa, surpassing existing solid-state barocaloric systems.
The practical performance was further assessed using a thermodynamic model of a Carnot-like cycle. The system delivered a cooling capacity of 67 J g−1 per cycle and 77% second-law efficiency at an 8-K temperature span, enabled by the extremely large cooling capacity and direct heat transfer of the solution.
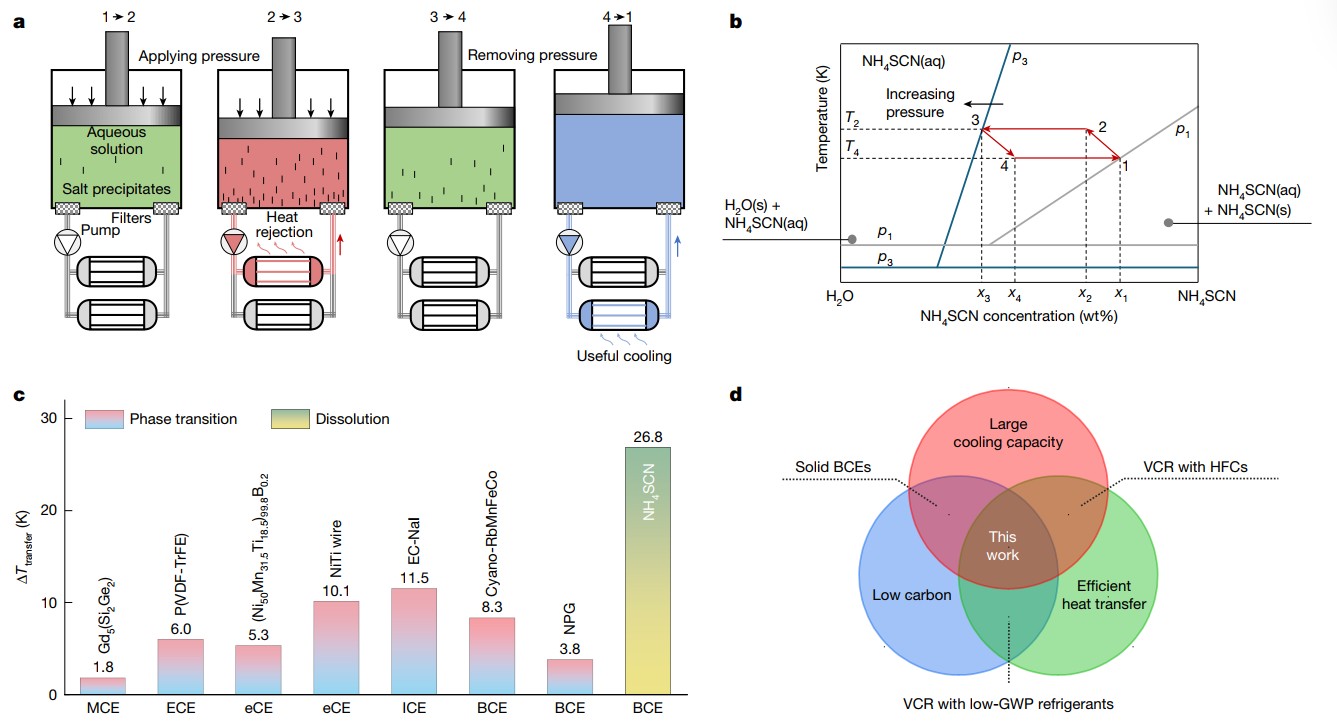
a) Schematic diagram of the Carnot-like cooling cycle with four steps using the NH4SCN aqueous solution. Unlike conventional solid-state caloric refrigerants that require external heat-transfer media, the present design enables direct heat transfer through solution circulation. The black arrows indicate that external pressure does work on the solution.
b) Carnot-like cooling cycle illustrated on the phase diagram of the NH4SCN aqueous solution. x1, x2, x3 and x4 are the NH4SCN concentrations at states 1, 2, 3 and 4, respectively; p1 and p3 are the pressures at states 1 and 3, respectively.
c) Comparison of the maximum ΔT transfer of NH4SCN at dissolution with state-of-the-art caloric materials, including magnetocaloric effect (MCE), electrocaloric effect (ECE), elastocaloric effect (eCE), ionocaloric effect (ICE) and BCE.
d) Venn diagram comparing the refrigeration technologies and their merits. Vapour-compression refrigeration (VCR) refrigerants with a low global warming potential (GWP) include R-1225ye(Z). EC-NaI, ethylene carbonate–sodium iodide; HFCs, hydrofluorocarbons; NPG, neopentyl glycol; P(VDF-TrFE), poly(vinylidene fluoride–trifluoroethylene).
For more information, the study has been published in Nature.
Researchers affiliations
Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, Shenyang, China
School of Materials Science and Engineering, University of Science and Technology of China, Shenyang, China
Center for High Pressure Science and Technology Advanced Research, Beijing, China
Japan Synchrotron Radiation Research Institute, Sayo, Japan
Department of Refrigeration and Cryogenic Engineering, Xi’an Jiaotong University, Xi’an, China
Key Laboratory of Materials Physics, Institute of Solid State Physics, HFIPS, Chinese Academy of Sciences, Hefei, China
Department of Physics, Southern University of Science and Technology, Shenzhen, China
Quantum Science Center of Guangdong-Hong Kong-Macao Greater Bay Area (Guangdong), Shenzhen, China
MOE Key Laboratory of Cryogenic Technology and Equipment, Xi’an Jiaotong University, Xi’an, China
Sources
[1] United Nations Environment Programme (2025). Global Cooling Watch 2025: The free degrees: How sustainable, passive-first cooling can save lives, money and food. Nairobi. https://www.unep.org/resources/report/global-cooling-watch-2025
[2] Navickaitė K. Additive manufacturing of regenerators for caloric cooling. https://iifiir.org/en/encyclopedia-of-refrigeration/additive-manufacturing-of-regenerators-for-caloric-cooling
[3] Rose R., Lawton R., Moya X. Barocaloric cooling and heating. Proceedings of the 26th IIR International Congress of Refrigeration: Paris, France, August 21-25, 2023. http://dx.doi.org/10.18462/iir.icr.2023.0572
[4] Zhang, K., Liu, Y., Gao, Y. et al. Extreme barocaloric effect at dissolution. Nature 649, 1180–1185 (2026). https://doi.org/10.1038/s41586-025-10013-1
Image credits: Institute of Metal Research (IMR), Chinese Academy of Sciences
Did you know? IIR has a working group dedicated to promoting international research collaboration in the areas of solid-state cooling, heat pumps, and energy harvesting.
