Cryopreservation techniques in reproductive medicine: improving cell survival after warming
A recent literature review presents the technical progress in cryopreservation techniques. The vitrification concept is presented as crucial to ensure cell survival after cryopreservation.
A recently published literature review provides an in-depth overview of the physical processes of cryopreservation. Cryopreservation of human gametes and embryos has taken a prominent place in the fertility treatment, especially for in vitro fertilisation (IVF). For instance, the freezing of surplus embryos has become necessary with the implementation of hormonal stimulation, which makes it possible to harvest of several oocytes per cycle. The development of various cryopreservation methods for application in assisted reproduction technology (ART) is constantly progressing.
According to the authors, from the very beginning, the main challenge in the development of cryopreservation techniques was to cool down the biological material from room temperature to −196 °C, while ensuring the cellular function and integrity of cell organelles and membranes after thawing.
The vitrification concept
lmost 80 years ago, Dr Basile Luyet uncovered key factors that could determine the viability of all cell types that undergo cryopreservation. According to Dr Luyet, the first cause of cell death was the change in the state of aggregation from liquid water into ice crystals in the intracellular compartment. To counteract the crystallization process, Luyet introduced the concept of vitrification. The general principle of vitrification is to transform a liquid into a glass-like amorphous solid that is free of any crystalline structures.
During the mid-1990s, the interest in vitrification started to grow in the context of ART, with increasing success in outcome.
The solidification of pure water in a glassy solid form (vitrification) is achieved when the temperature decreases extremely rapidly below the glass transition temperature (Tg; Figure 1). It has been found that the Tg for pure water is −137 °C and is only possible at cooling rates (C) exceeding 100,000 °C/min to avoid spontaneous crystal nucleation when crossing the zone between Tm (melting temperature) and Tg. At slow cooling rates, the formation of crystals is directly observed: pure water takes on a milky colour. Conversely, at extremely high cooling rates, a glassy solid state is observed when the pure water reaches Tg: pure water then has a transparent appearance.
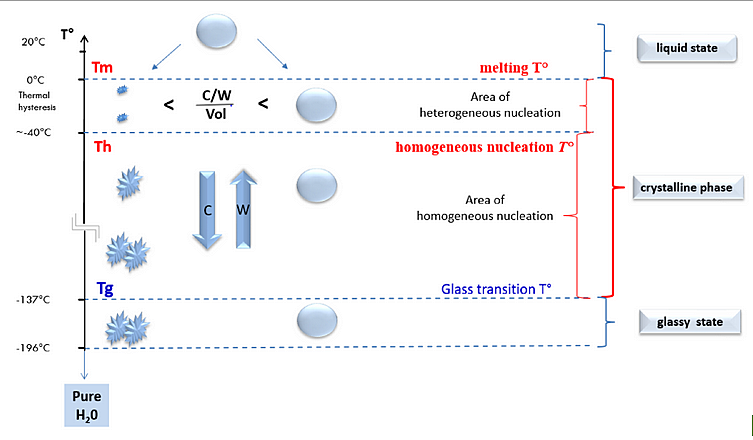
According to the authors, research has shown that biological material cannot survive at very low temperatures without the usage of water-soluble cryoprotective agents (CPA). CPAs increase viscosity and thereby slow down the molecular movements of water. The result is a reduction in the growth rate of ice crystals and a limitation of the size of the crystals, among other effects. The authors noted that cryopreservation solutions containing salts, amino acids, proteins, and CPAs have different phase change temperatures. The shift in these parameters depends on the respective concentration and composition of solved molecules and CPAs.
The fundamental issue in all vitrification methods is to achieve and maintain conditions inside and outside the cells that guarantee an amorphous state throughout the cooling process, as well as during the warming process. This is reached when the solutes are sufficiently concentrated, or when cooling is of sufficient speed, so that the increase in viscosity inhibits nucleation and prevents the growth of ice crystals.
Intracellular vitrification: a key factor for successful cryopreservation procedures
There are several approaches to gametes (oocytes or spermatozoa) and embryos cryopreservation (see table 1):
- the conventional vitrification protocol using a high concentration of permeable CPAs and high cooling/warming rates;
- the slow freezing protocol with the application of substantially lower concentrations of permeable CPAs and low cooling rate;
- the directional freezing with permeable CPAs and precise control of the solution effect;
- the spermatozoa cryopreservation method without using permeable CPAs.
Before cooling gametes or embryos down to −196°C in liquid nitrogen, the intracellular compartment must be prepared to allow the achievement and maintenance of an intracellular vitreous state. To reach this objective, the biological material is exposed to gradually increasing concentrations of non-vitrifying solution (nVS) and vitrifying solution (VS).

Regardless of the protocol used for cryopreservation, the common denominator of cell survival after cryopreservation is the achievement of an intracellular colloidal vitrified state. In an aqueous solution, a glassy vitrified state can be observed following ultra-fast cooling. In contrast, the intracellular compartment will be vitrified, due to a huge increase in viscosity after dehydration, macromolecular crowding, and uptake of CPA.
For more details on the underlying chemical and physical principles, the complete study is available in FRIDOC.
Source
Vanderzwalmen, P.; Ectors, F.; Panagiotidis, Y.; Schuff, M.; Murtinger, M.; Wirleitner, B. The Evolution of the Cryopreservation Techniques in Reproductive Medicine—Exploring the Character of the Vitrified State Intra- and Extracellularly to Better Understand Cell Survival after Cryopreservation. Reprod. Med. 2020, 1, 142-157. https://doi.org/10.3390/reprodmed1020011
